Industry Insights
Home > News > Industry Insight > WecLac® Bifidobacterium animalis subsp. lactis BLa80 Regulates Intestinal Habits in Adults with Chronic Constipation
WecLac® Bifidobacterium animalis subsp. lactis BLa80 Regulates Intestinal Habits in Adults with Chronic Constipation

1. Introduction
Chronic idiopathic constipation (CIC) is a common gastrointestinal disorder characterized by infrequent bowel movements, difficult stool passage, and incomplete evacuation. It significantly impacts patients' quality of life and is more prevalent among women. Despite various treatments, some patients fail to respond to conventional therapies, prompting interest in alternative solutions such as probiotics.
Bifidobacterium animalis subsp. lactis BLa80, a probiotic strain isolated from human breast milk, has shown potential in preclinical studies for treating gastrointestinal issues. This multicenter, randomized, double-blind, placebo-controlled clinical trial investigates the efficacy and safety of B. animalis subsp. lactis BLa80 in regulating bowel habits in adults suffering from chronic constipation.
2. Study Design
The study enrolled 46 adults aged 18 and older with chronic constipation, defined as having fewer than three stools per week or stool types 1-2 on the Bristol Stool Chart. Participants were randomly assigned to receive either B. animalis subsp. lactis BLa80 in maltodextrin or a placebo for 12 weeks. The study was conducted at three Spanish primary care centers.
Primary endpoints included changes in stool consistency and weekly stool frequency, evaluated at weeks 4, 8, and 12. Secondary outcomes assessed constipation-related symptoms (PAC-SYM), quality of life (PAC-QoL), and gastrointestinal well-being (GI-QLI).
3. Results
· Stool Consistency: Patients receiving B. animalis subsp. lactis BLa80 showed significant improvement in stool normalization, shifting from Bristol types 1-2 to 3-4. At week 8, stool normalization was significantly better in the BLa80 group (P=0.006) compared to the placebo, with further improvement by week 12 (P=0.027).
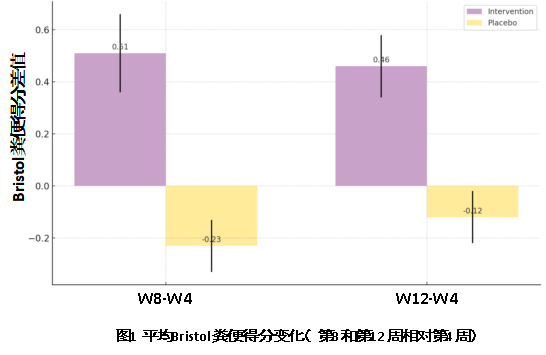
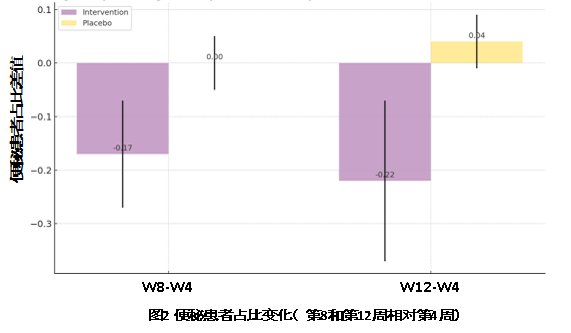
· Stool Frequency: The probiotic intervention group showed a significant reduction in constipation, decreasing by 0.17 and 0.22 at weeks 8 and 12. The placebo group had minimal changes, with no improvement at week 8 and a slight increase (0.04) at week 12.
· Constipation Symptoms: No significant differences were found in the PAC-SYM scores between the two groups, indicating similar levels of improvement in symptoms like abdominal pain, bloating, and swelling.
· Quality of Life: The PAC-QoL and GI-QLI questionnaires showed no significant differences in quality of life improvements between the two groups throughout the study.
· Safety: The probiotic was well tolerated, with no significant adverse effects reported in the BLa80 group.
4. Discussion
The study confirms the efficacy of B. animalis subsp. lactis BLa80 in improving stool consistency in patients with chronic constipation, with significant improvements seen by week 8. However, changes in stool frequency and symptom relief were comparable between the probiotic and placebo groups.
The probiotic's role in stool normalization suggests a promising adjunctive therapy for constipation management, potentially enhancing the effects of other treatments. Further research is required to explore optimal dosing and treatment duration, as well as the long-term effects of B. animalis subsp. lactis BLa80 on gut health.
5. Conclusion
The oral administration of Bifidobacterium animalis subsp. lactis BLa80 in maltodextrin for 12 weeks significantly improved stool consistency in adults with chronic constipation. The treatment was well-tolerated and could be considered a safe and effective option for improving digestive health. Further studies are needed to fully understand the benefits of this probiotic and its potential as part of a comprehensive treatment approach for chronic constipation.







 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin