Industry Insights
Clinical Study: Bifidobacterium animalis subsp lactis BLa80
Clinical Study: Bifidobacterium animalis subsp lactis BLa80 Enhances Symptoms of Constipation-Predominant Irritable Bowel Syndrome
Clinical summary
(1) To date, there is no effective treatment for irritable bowel syndrome with constipation (IBS-C), a condition characterized by significant constipation symptoms. Probiotics have shown potential in alleviating symptoms associated with IBS-C.
(2) The aim of this clinical efficacy study, conducted by the MicroCare Probiotics Research Institute in collaboration with healthcare providers, was to evaluate the impact of a 4-week intervention of probiotic Bifidobacterium animalis subsp. lactis BLa80 supplementation on defecation patterns, IBS-related symptoms, and quality of life in IBS-C patients. The study followed a parallel, double-blind, randomized, controlled trial design.
(3) The results of this study demonstrated that Bifidobacterium animalis subsp. lactis BLa80 effectively relieved IBS-related symptoms in patients with IBS-C. This intervention showed a positive trend towards improved bowel frequency and a significant reduction in the severity of IBS symptoms.
Clinical Study on Bacterial Strains
Study Objective
The aim of this study was to evaluate the impact of Bifidobacterium animalis subsp. lactis BLa80 on defecation, gastrointestinal symptoms, and quality of life in patients with irritable bowel syndrome with constipation (IBS-C).
Study Methods
The study involved a total of 180 participants who met the inclusion criteria of being between the ages of 18-70 years and having a BMI between 18.5-30 kg/m2. The study involved a 4-week oral probiotic treatment.
Visits 1-4
During the study, participants were required to complete a research questionnaire on a daily basis and record bowel movements, including frequency and phenotype.
The intervention included the following treatments:
Placebo: 2.0g maltodextrin/day
Probiotics: 2.0g BLa80 800 billion CFU/day
Probiotics: 2.0g AF supplement/day
Intervention with Bifidobacterium animalis subsp. lactis BLa80 resulted in a trend towards improved bowel frequency
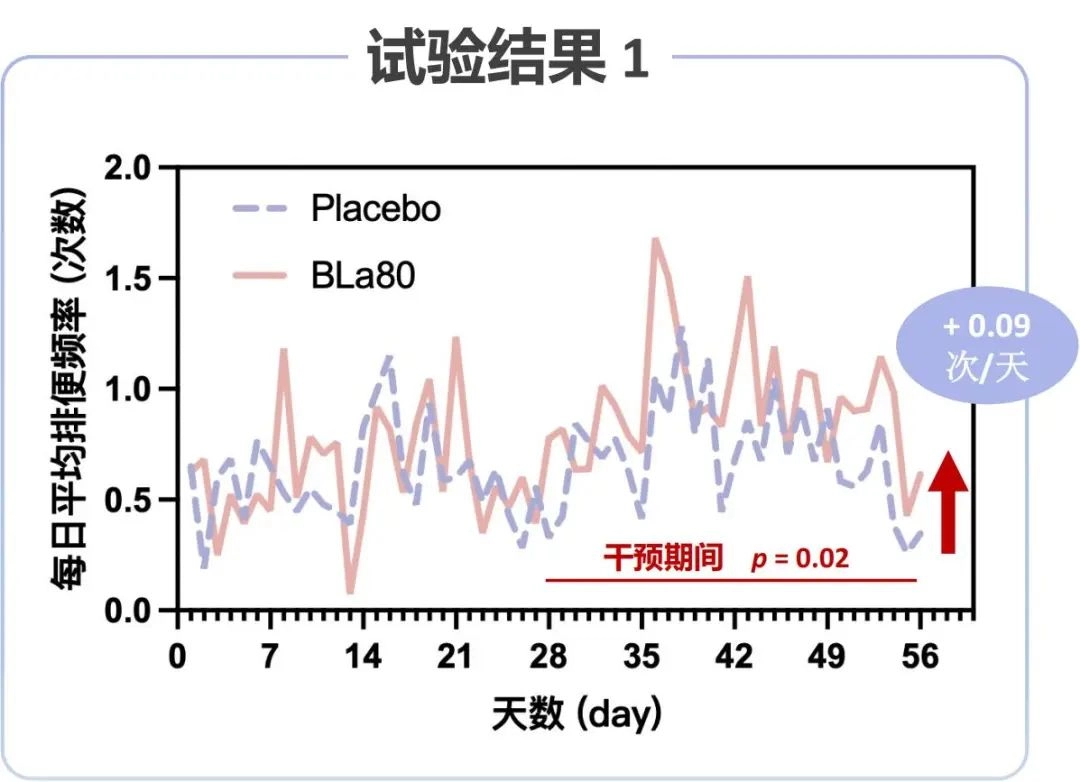
During the 8-week clinical intervention period, patients who were supplemented daily with probiotic BLa80 showed a trend toward increased fecal frequency compared to IBS patients in the placebo group (p=0.08). For patients with IBS-C, a significant reduction in irritable bowel syndrome symptoms was observed when the frequency of defecation was increased. Additionally, questionnaires revealed improved patient anxiety and depression. These results suggest that the BLa80 intervention improves the frequency of defecation in patients with IBS.
Bifidobacterium animalis subsp. lactis BLa80 Intervention Results in Significant Reduction in IBS Symptom Severity
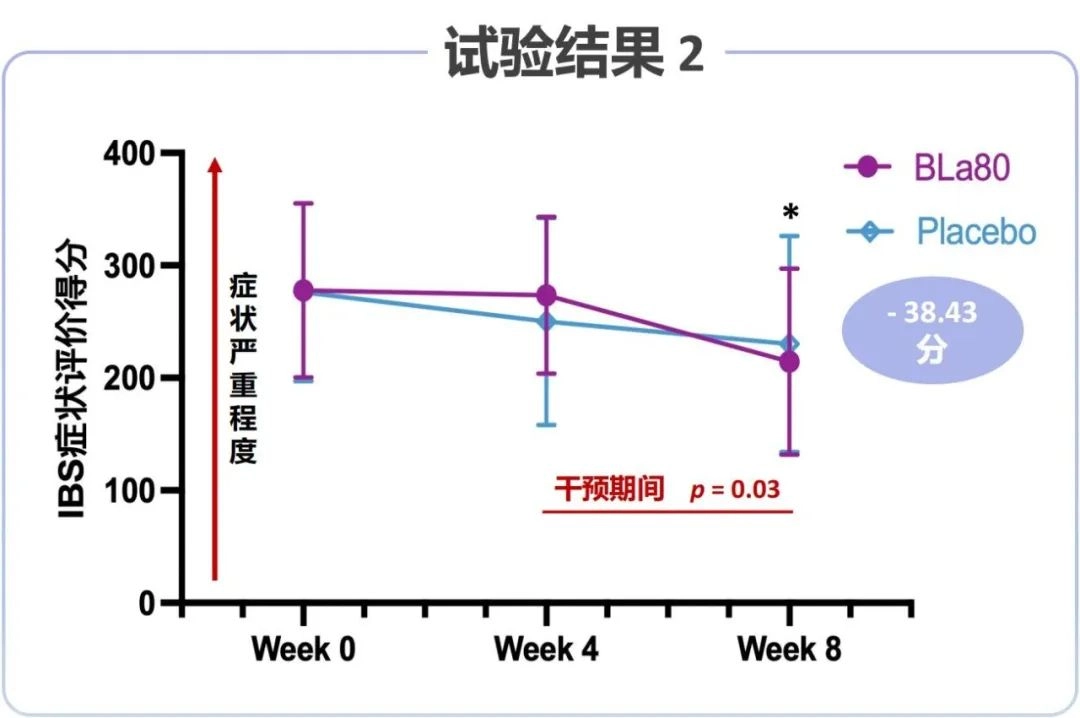
As the clinical observation time increased, patients with IBS in both the placebo and probiotic BLa80 groups showed a reduction in the severity of IBS symptoms. However, the effect of the BLa80 intervention was superior to that of the placebo group, and was more pronounced at week 4 recordings. This may be due to the rapid improvement in intestinal flora of the patients for a short period of time after intake of the probiotic. The IBS symptom severity scores of the BLa80 group were lower at week 8 recordings, suggesting that the probiotic sustained its effect and was well-tolerated.
Study Conclusion
The ingestion of BLa80 probiotic supplementation during the intervention period resulted in a significant reduction in irritable bowel syndrome symptom severity and a trend towards improved bowel frequency compared to placebo. Overall, all BLa80 probiotics were well-tolerated by IBS patients with fragile bowel. Daily dietary supplementation with probiotics may be beneficial for patients with IBS-C by alleviating IBS-related symptoms, particularly those related to bowel frequency and symptom severity.







 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin