Industry Insights
Home > News > Industry Insight > WecMixTM Probiotic Formulation Containing WecLac® Lactobacillus acidophilus LA85 and Bifidobacterium animalis subsp. lactis BLa80 in Inhibiting Helicobacter pylori
WecMixTM Probiotic Formulation Containing WecLac® Lactobacillus acidophilus LA85 and Bifidobacterium animalis subsp. lactis BLa80 in Inhibiting Helicobacter pylori
Abstract
The Research focuses on the use of the probiotic strains Lactobacillus acidophilus LA85 and Bifidobacterium animalis subsp. lactis BLa80 to inhibit the growth of Helicobacter pylori (H. pylori). The combined strains demonstrate significant inhibition zones of 25mm and 16mm respectively. Moreover, both strains reduce H. pylori's urease activity and its adhesion to AGS cells. Animal model experiments show that a mixture of LA85 and BLa80 can alleviate the inflammation caused by H. pylori infection. The combination of these strains proves more effective in relieving inflammation than either strain alone.
Introduction
H. pylori is a Gram-negative bacterium known to colonize the human stomach. It has been implicated as a major cause of chronic gastritis, peptic ulcers, and gastric cancer. The current treatment regimen involves antibiotics, which may result in resistance and gut microbiome disruption. This invention proposes a natural, probiotic-based alternative to mitigate H. pylori-related diseases.
Probiotic Strains Used
· Lactobacillus acidophilus LA85: A strain with high resistance to gastrointestinal stress, inhibiting common pathogens such as Escherichia coli and Staphylococcus aureus. LA85 has no antibiotic resistance and is effective in regulating immune responses.
· Bifidobacterium animalis subsp. lactis BLa80: This strain shows excellent bile salt hydrolase activity, effectively lowering serum cholesterol levels and modulating gut microbiota.
Mechanism of Action
1. Inhibition of H. pylori Growth:
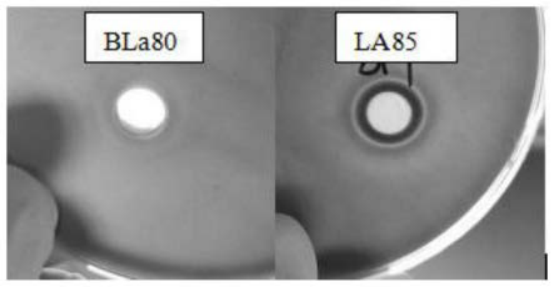
o LA85 and BLa80 inhibit the growth of H. pylori, with respective inhibition zone diameters of 25mm and 16mm.
2. Reduction in Urease Activity:
o Urease activity in H. pylori is crucial for its survival in the acidic environment of the stomach. LA85 inhibits urease by 78.63%, while BLa80 inhibits it by 67.49%.
3. Adhesion to AGS Cells:
o The adhesion of H. pylori to gastric epithelial cells is reduced by both strains. LA85 reduces H. pylori adhesion to AGS cells by 30.62%, and BLa80 reduces it by 37.87%.
Experimental Validation
1. In Vitro Studies:
o The strains were tested for their ability to form inhibition zones against H. pylori. Inhibition zone diameters were measured using a disc diffusion method on Columbia agar plates.
2. Animal Models:
o A mouse model was used to study the anti-inflammatory effects of the probiotic mixture. Mice infected with H. pylori were treated with a mixture of LA85 and BLa80, which significantly reduced the levels of pro-inflammatory cytokines IL-6 and IL-1β.
3. Human Trials:
o A small-scale clinical trial was conducted with 42 H. pylori positive individuals. The group treated with a probiotic mixture of LA85 and BLa80 showed a significant reduction in gastrointestinal symptoms and a higher eradication rate of H. pylori compared to the placebo group. The eradication rate in the probiotic group was 72.72%, compared to 15% in the placebo group.
Applications
The combination of Lactobacillus acidophilus LA85 and Bifidobacterium animalis subsp. lactis BLa80 has potential applications in:
· Functional Foods: These probiotics can be incorporated into beverages, tablets, and dairy products designed to alleviate gastrointestinal discomfort and prevent H. pylori-related conditions such as ulcers and gastritis.
· Pharmaceuticals: The strains can be formulated into powders, capsules, or tablets for the treatment of H. pylori infections and related inflammatory diseases.
Conclusion
This patent demonstrates that the probiotic strains LA85 and BLa80 exhibit promising potential in the prevention and treatment of H. pylori infections. Their combined use enhances the therapeutic effects, providing a natural alternative to antibiotic therapy.








 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin