Industry Insights
Home > News > Industry Insight > Clinical trial of WecLac® Weizmannia coagulans BC99 in Alleviating Chronic Constipation
Clinical trial of WecLac® Weizmannia coagulans BC99 in Alleviating Chronic Constipation

Introduction
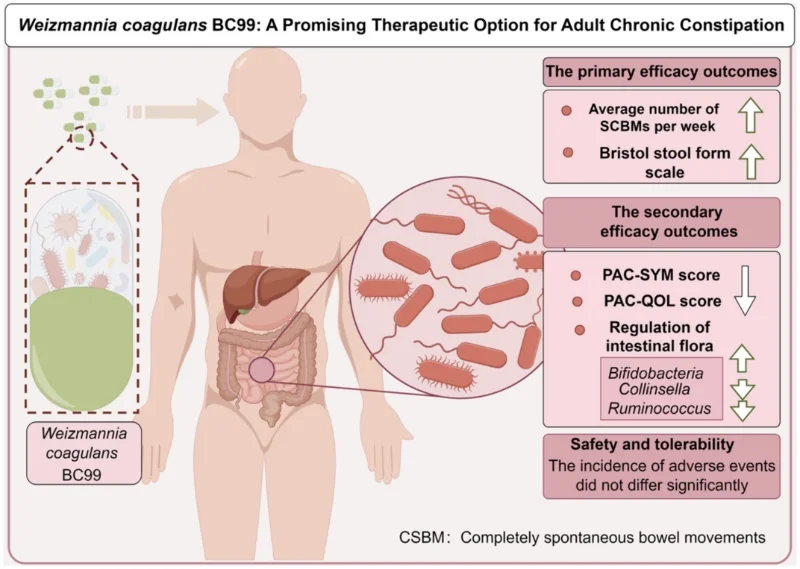
Weizmannia coagulans BC99, a novel probiotic strain, has shown potential in treating gastrointestinal disorders, particularly chronic constipation. Chronic constipation, a common ailment affecting around 16% of the global population, severely impacts the quality of life. This clinical study investigates the efficacy and safety of Weizmannia coagulans BC99 in alleviating chronic constipation in adults.
Study Design
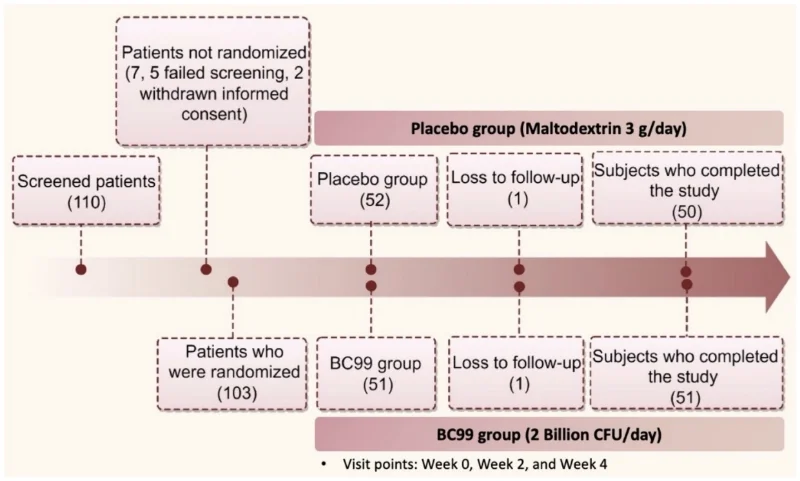
This study was a randomized, double-blind, placebo-controlled trial conducted over four weeks. Participants were randomly assigned to receive either Weizmannia coagulans BC99 (2 billion CFU/day) or a placebo (maltodextrin). The study was approved by the Ninth People’s Hospital of Suzhou and followed the Helsinki Declaration and Good Clinical Practice guidelines.
Inclusion Criteria:
· Chinese adults aged 18-65 years
· Diagnosed with chronic constipation (Rome IV criteria)
· Willingness to comply with study procedures and signing of informed consent
Exclusion Criteria:
· Presence of systemic conditions like diabetes or cancer
· Recent use of antimicrobials, probiotics, or drugs affecting gastrointestinal motility
· Pregnancy or breastfeeding
· Severe cardiovascular, cerebrovascular, liver, kidney, hematological, endocrine diseases, or mental disorders
A total of 110 participants were screened, with 103 starting treatment and 101 completing the study.
Efficacy Assessment
Participants recorded daily bowel-related symptoms. The primary and secondary efficacy endpoints were measured through various scales and laboratory tests.
Primary Endpoints:
· Increase in spontaneous complete bowel movements (SCBM) per week
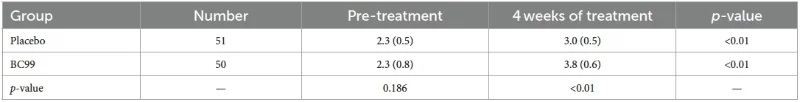
Comparison of the average number of SCBMs per week for 4 weeks of treatment between the two groups.
· Stool consistency according to the Bristol Stool Form Scale
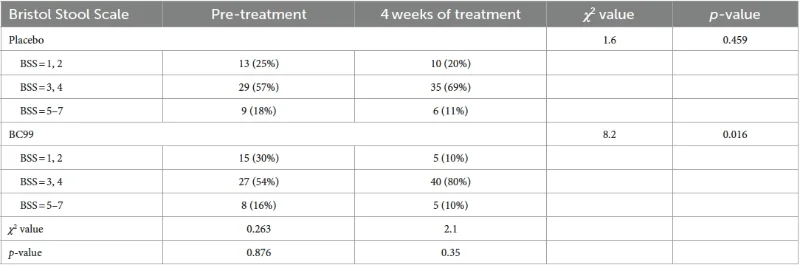
Comparison of Bristol scores of fecal traits between the two groups at 4 weeks of treatment
Secondary Endpoints:
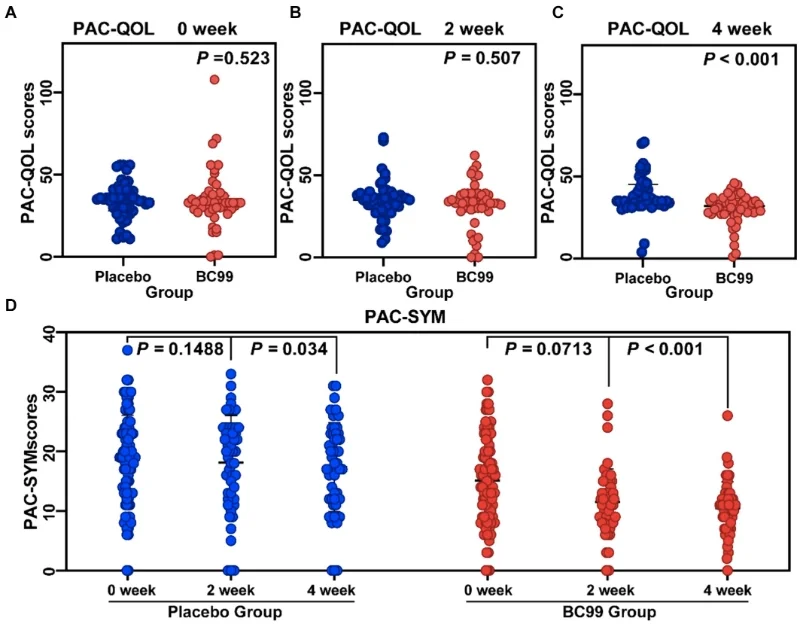
· Quality of life measured by Patient Assessment of Constipation Quality of Life (PAC-QOL)
· Changes in serum IL-6 levels
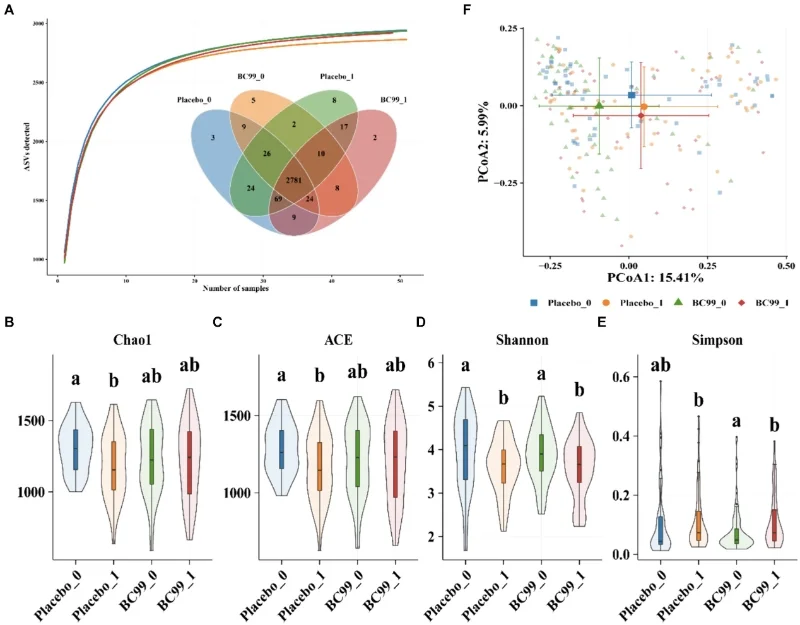
· Gut microbiota composition analyzed via 16S rRNA gene sequencing
Results
After 4 weeks, the Weizmannia coagulans BC99 group showed significant improvements:
· Increased SCBM (p = 0.002)
· Reduced PAC-QOL scores (p < 0.001)
· Lower PAC-SYM scores (p < 0.001)
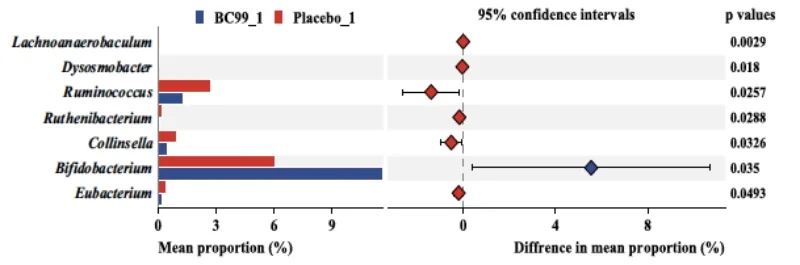
Additionally, BC99 modulated key gut microbiota such as Bifidobacterium and Ruminococcus, linked to crucial metabolic pathways like glutathione metabolism. Safety monitoring indicated no adverse effects, confirming BC99 as a safe therapeutic option.
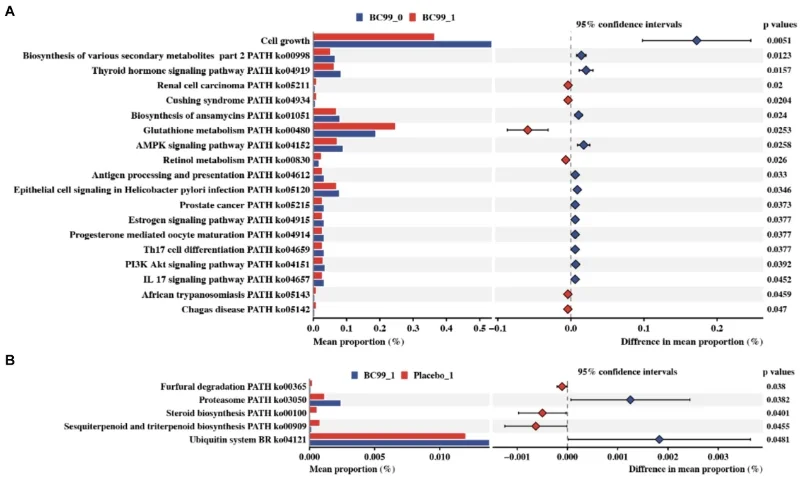
Discussion
The trial demonstrated that Weizmannia coagulans BC99 effectively alleviates chronic constipation symptoms, enhances gut microbiota balance, and influences critical metabolic pathways. This probiotic strain presents a promising alternative to traditional pharmacotherapies, with significant implications for gastrointestinal health management.
Conclusion
Weizmannia coagulans BC99 is an effective and safe therapeutic option for relieving chronic constipation in adults. This study supports further exploration of BC99 in broader biomedical applications, potentially transforming probiotic therapy in gastrointestinal health.
References
Wu Y, Bai Z, Jin Y, Zhu H, Dong Y, Gu S, Jin Y. (2024) A randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Weizmannia coagulans BC99 in the treatment of chronic constipation in adults. Frontiers in Nutrition. 11:1395083.
doi: 10.3389/fnut.2024.1395083.







 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin