Industry Insights
Home > News > Industry Insight > Clinical Study--WecLac® B. animalis subsp. lactis BLa80 Adjuvant Quadruple Therapy
Clinical Study--WecLac® B. animalis subsp. lactis BLa80 Adjuvant Quadruple Therapy
Clinical summary
[1] The current clinical treatment of Helicobacter pylori mainly uses a combination of antibiotics, which is prone to recurrence. In addition, irrational use of antibiotics can cause some adverse reactions.
[2] This clinical efficacy study was carried out by the Wecare Probiotics Research Institute in cooperation with relevant medical institutions. The purpose is to evaluate the conventional quadruple therapy combined with the probiotic Bifidobacterium animalis subsp lactis BLa80 in the treatment of Helicobacter pylori (Hp) infection, and the Clinical efficacy of adverse drug reactions during antibiotic treatment.
[3] This study proves that Bifidobacterium animalis subsp lactis BLa80 has a good adjuvant therapeutic effect in the eradication of Helicobacter pylori (Hp) quadruple therapy, significantly improving the severity of patients' gastrointestinal symptoms and significantly reducing the risk of drug treatment—incidence of nausea as an adverse reaction.
Strain clinical research
Research purpose: Effect of BLa80 adjuvant quadruple therapy on gastrointestinal adverse reactions in the eradication treatment of Helicobacter pylori (H. pylori)
Research methods
● Subjects: 100 cases in total
● Inclusion criteria: Age 18-70 years old, 13C or 14C-UBT positive
● Oral probiotic treatment for 2 weeks
Visits 1-4:
● Fill out the questionnaire: GSRS rating scale
● Collect stool samples for sequencing

Placebo: bismuth quadruple + 3.0g maltodextrin/Day
Probiotics: Bismuth quadruple + 3.0g BLa80 10 billion CFU/Day
● Bifidobacterium animalis subsp lactis BLa80 significantly improved the patient’s GSRS score and improved the patient’s gastric discomfort symptoms
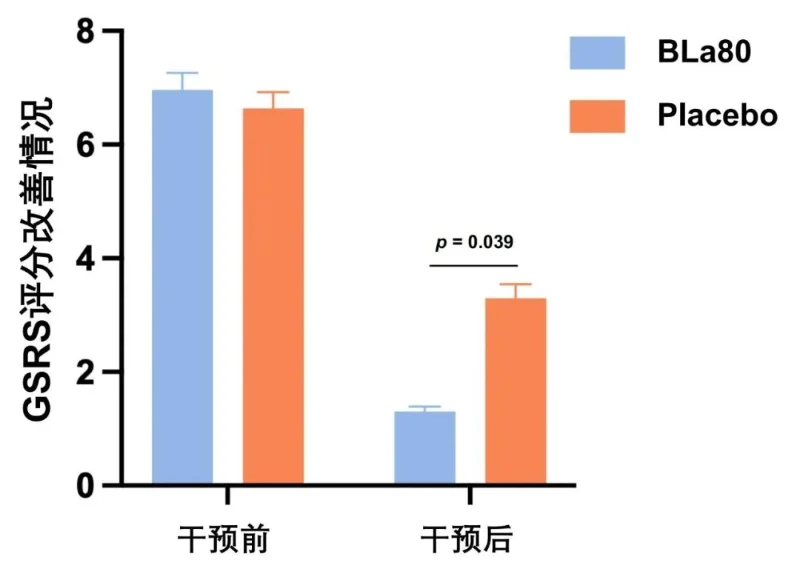
The total GSRS score (severity of gastrointestinal symptoms) of the patients after H. <0.001).
● Bifidobacterium animalis subsp lactis BLa80 significantly reduces the incidence of gastrointestinal adverse reactions (nausea) during drug treatment
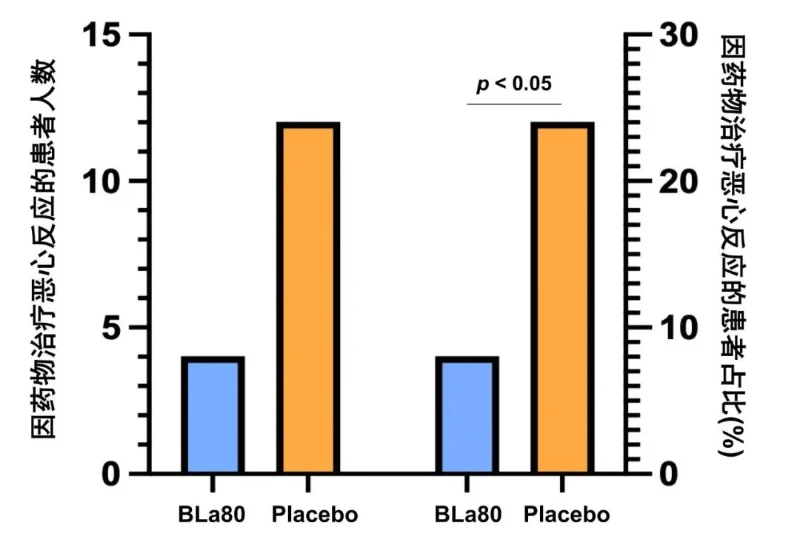
Analysis conclusion
In the eradication treatment of Helicobacter pylori, the probiotic bifidobacterium animalis subsp lactis BLa80 adjunct to conventional quadruple therapy showed a high eradication rate. At the same time, after probiotic BLa80 was used as an adjuvant treatment for Hp, the patient's gastrointestinal symptom rating score (GSRS) was significantly lower than that of the placebo group, significantly improving the patient's clinical symptoms (p < 0.05), and patients in the probiotic BLa80 group experienced symptoms during treatment. The incidence of nausea was significantly lower than in the placebo group (p < 0.05), indicating that adding probiotic BLa80 may help reduce adverse effects caused by conventional antibiotic treatment.





 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin